Metallic Biomaterials
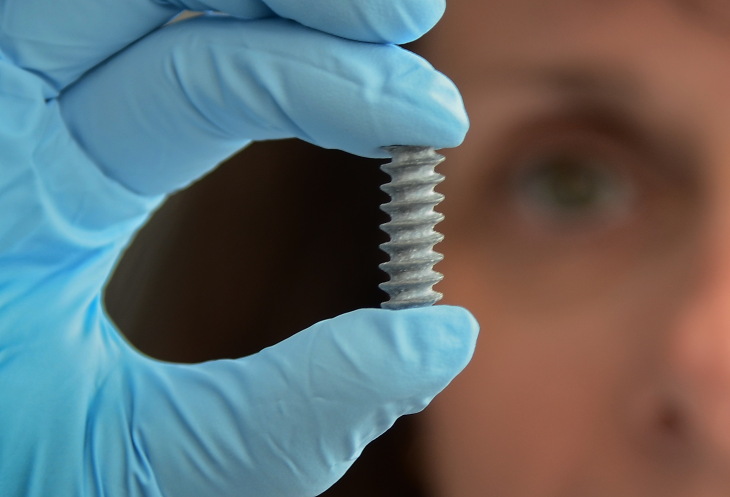
Photo: Carsten Neff
Broken bones do not always heal as wished. Then doctors have to assist: they fix the damaged bones with screws, pins or plates so that it can grow back together. Until now, these implants have been made from stainless steel or titanium. But once the bone has healed, they can be troublesome, for instance as a potential source of infection. This is why implants are sometimes removed. For the patient, this means an additional operation – including a stay in hospital, potential pain and time off work.
For this reason, researchers in the Institute of Metallic Biomaterials in Geesthacht are working on implants that dissolve automatically in the body once the bones have grown back together. This saves patients a further stressful intervention. And should the bone break again at a later date, doctors can treat it quite normally – the point of the break will have completely regenerated in the intervening time.
Overview
Implants
A promising candidate for use in dissolving implants is the light metal magnesium. On the one hand, it is strong enough to be used as a screw to fix a damaged bone. On the other, it disintegrates over time through the actions of body fluids and the immune system and gradually dissolves. Magnesium is not a foreign substance for the body: every human body naturally contains around 25 grams of it, the majority as an important mineral in the bones.
At the same time, the human organism has the ability to efficiently break down excessive magnesium. Plus this light metal has, for many years, been licensed as a dietary supplement – and can be found in any drugstore.
It's the (ad)mixture that matters
In the laboratory, test series are carried out using magnesium. Photo: Hereon/Christian Schmid
Since 2013, the Hannover Medical School (MHH) has offered a method to treat hammer toes or bunions (Hallux valgus), an often painful deformation of a toe bone. During the operation, the bone is reshaped and fixed into position using a magnesium screw. However, these screws are only suitable for slight to medium deformations and appear to disintegrate relatively slowly. More ideal would be systems, that could also be used for serious bone defects and would dissolve quickly after healing.
To develop such robust yet degradable implants, the Helmholtz-Zentrum Hereon has set up a comprehensive process and test chain. Pure magnesium is not very suitable for implants. This is why researchers are mixing it with other materials, such as small amounts of the elements gadolinium or yttrium. These alloying elements adjust the material properties in a specific way: how stable is the implant, and how long will it last until it decomposes in the body? Other admixtures could have an effect on the organism: silver has an anti-bacterial effect, calcium supports bone growth.
Intensive testing of magnesium

The material is subjected to numerous test series in the laboratory. Photo: Hereon/Christian Schmid
To produce the workpieces, the prototypes are cut from magnesium rods or plates. Alternatively, the experts mix fine powder with a plastic binder to form a type of modelling material that is then injected into a mould and subsequently “sintered” in special ovens. Here the particles bake solidly together to form the required component, such as a screw. Using this new powder-metallurgical procedure, prototypes can be produced in a way that protects resources – there is hardly any production waste.
Once the component is produced, the properties of the alloy are put through their paces. Test machines load the prototypes and measure values such as the hardness and strength. Special procedures developed in-house identify how the material decomposes in environments similar to those present in the body – in nutrient solutions with salts, vitamins and proteins, and at temperatures of 37 degrees Celsius. Material systems that prove themselves in the test series are explored further.
Extensive tests follow, using live cell cultures. Here the experts find out how happy the cells are in contact with the alloy, how well they thrive and the extent to which the decomposing magnesium changes the metabolism. The surprising finding: whilst the cells in individual tests react relatively sensitively to high concentrations of magnesium, they are significantly less sensitive in communities. The cells appear to coordinate and develop a joint response to the material.
A close medical network

The prototype of a bone screw made from magnesium. Photo: Hereon/Thomas Ebel
A few alloys which seem the most interesting to researchers will finally also be studied in animal models in trials on rats and mice. To obtain the greatest amount of data from the fewest possible animal experiments, scientists are using numerous modern analysis procedures – from computer tomographs and MRI scanners to detailed examinations of tissue sections and bone samples. To do this, they are working closely together with various university hospitals – for instance within the scope of the “Molecular Imaging North Competence Center” (MOIN CC) in Kiel or the virtual “MetBioMat”, in which the university hospitals of Hamburg, Hannover and Graz are involved.
Results so far are promising. But before they use the new materials, the scientists need a sound understanding of the highly-complex processes and mechanisms: how, in detail, does the immune system respond to specific alloys? To what extent can the released magnesium actually promote bone formation? The more accurately these questions can be answered, the better the chance of achieving the ideal aim: implants that dissolve quickly, but only once the bone has completely healed.
