Hydrogen
- Hydrogen Storage Materials
- Development and Characterisation of novel Materials for Hydrogen Storage
- Development of Hydrogen Storage Tanks
Hydrogen fuel cell car of the Helmholtz-Zentrum Hereon.
Within the Helmholtz “Advanced Engineering Materials” Programme, we are developing nanostructured materials for hydrogen storage, are investigating possible large-scale production routes and are testing these materials under application-oriented conditions.
Solid-state hydrogen storage – based on light metal hydrides or hydride composites – enables us to create an extremely safe storage system for use in future zero-emission vehicles and in chemical energy storage. Such systems will also have to provide high capacity storage with respect to volume and weight. In contrast to gaseous hydrogen storage in high-pressure tanks and to liquid hydrogen storage, hydrogen in solid-state storage is bound in the material during uptake, thereby releasing heat.
Hydrogen Storage Materials
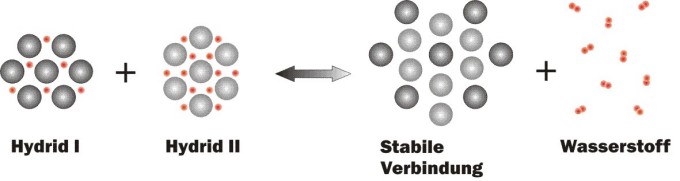
Scheme of the reactions taking place in a "Reactive Hydride Composite"
The amount of heat released in this process has to be supplied to the tank again during the release of the bound hydrogen. Solid-state hydrogen storage, in comparison to high pressure and liquid hydrogen storage, is the most energy efficient means of storing hydrogen as well as the only one without additional energy losses (energy required for compression, liquefaction, etc.). This efficiency can be achieved if we succeed in gaining the energy needed to release the hydrogen from process heat that cannot be used otherwise. In order to achieve this, it is necessary to adapte or tailore the reaction enthalpy/heat of the storage material to the available process (waste) heat (for example, waste heat from a fuel cell or combustion engine).
The graph below shows that under such conditions, storage in metal hydrides can also be cost-efficient when taking into consideration the cost over the entire life-cycle, including hydrogen production and processing for storage.
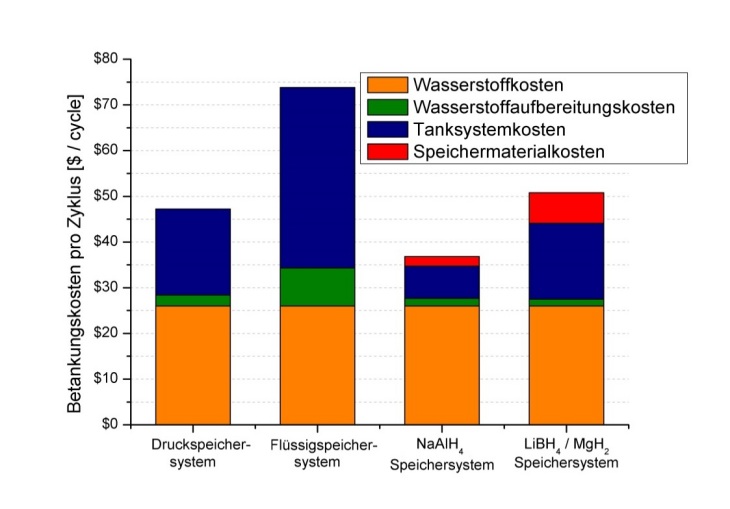
Cost distribution of various types of hydrogen tanks on one charge (individual tank loading and unloading) with a storage amount of 4kg of hydrogen (from ). Especially the sodium alanate (NaAlH4)-based solid-state storage systems prove to have significant cost advantages due to lower tank and hydrogen processing cost compared to pressure and liquid hydrogen storage systems when taking into consideration the entire life-cycle of the tank (calculated here with 1500 charge cycles, which is a typical number of cycles in the automotive sector).. (J. Jepsen, et al., "Economic potential of complex hydrides compared to conventional hydrogen storage systems.", Int. Journal of Hydrogen Energy. 37 (5), (2012) S. 4204 - 4214. DOI: 10.1016/j.ijhydene.2011.11.141)
With the discovery of the “Reactive Hydride Composites” in 2004, researchers at the Helmholtz-Zentrum Hereon and the HRL Laboratories, independently of each other, made a crucial breakthrough. Both groups could demonstrate that the reaction heat use/release in such composite materials can be considerably altered in comparison to pure hydrides.
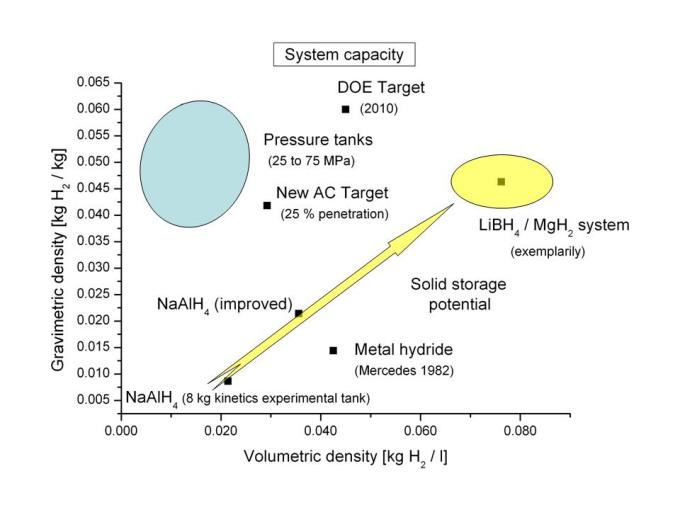
Technical potential of Solid State Hydrogen Storage
The aim of our current work is the further optimization of reaction kinetics for these new materials, their cost-efficient production and their qualification for technical implementation.
- the study of light metal hydrides, complex hydrides and Reactive Hydride Composites as well as of suitable catalysts that can provide hydrogen storage densities and kinetics tailored to application
- the low cost production of nanocrystalline hydrogen storage materials using high-energy milling and development of a technological process route for industrial use
- optimization of tank design for complex hydrides and Reactive Hydride Composites
We pursue these objectives within the framework of the Helmholtz "Advanced Engineering Systems" Programme as well as in a number of European and nationally funded projects that are often coordinated by us; we also carry out contract research in close collaboration with the industry.
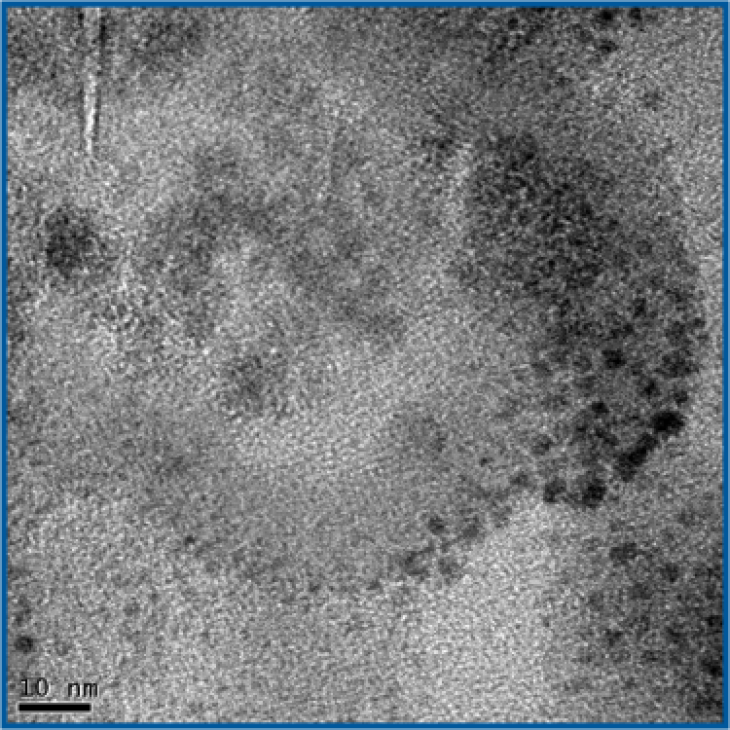
Transmission electron microscopy of the nanostructure of a Reactive Hydride-Composite. black „balls“ could be identified as catalyst particles.
Scientists at the Helmholtz-Zentrum Hereon have been working on the development and optimization of light metal hydrides since 1998. They initially found magnesium especially interesting due to its vast availability, low cost and high storage capacity, but they encountered two serious drawbacks:
- Even at temperatures of over 300°C, the reaction rate was far too slow for the uptake and release of hydrogen
- An excessively high reaction enthalpy or reaction heat that needs to be provided or cooled during the release or the uptake of hydrogen, respectively.

Metallhydride for hydrogen storage
By high-energy milling as well as the discovery of the reaction-accelerating influence of different transition metal oxides in 1999 at the former GKSS Research Centre (now the Helmholtz-Zentrum Hereon), the reaction rate of Mg or MgH2 with hydrogen was improved so drastically that the loading of Mg with hydrogen is now possible within a few minutes even at room temperature.
Reducing the heat of the reaction, resulting from the strong Mg – H bond, produced a much greater problem because any addition (alloying) to Mg in the past always also lead to a significant reduction in the weight-related storage capacity. It was only in 2004 that three independent research groups at the Helmholtz-Zentrum Hereon, the HRL Laboratories in the United States and the Korean Institute of Science and Technology (KIST) managed, by introducing Mg-based reactive hydride composite materials, to not only significantly reduce the reaction heat but to also have an even higher weight-related storage capacity for hydrogen than pure Mg, with a maximum reversible storage capacity of 11 % by weight.
The activities for the development and optimization of storage materials at the Helmholtz-Zentrum Hereon currently focus on:
- the development of advanced storage materials with high capacity and moderate to low operating temperatures
- reaction rate optimization of the discovered Reactive Hydride Composites
- investigation of particularly cost-effective storage materials
The scientists at the Helmholtz-Zentrum Hereon have been working on the production of nanostructured materials using the high-energy milling process since 1992.
Upscaling the production process from the laboratory to the industrial scale has made up an important part of our research activity since 2000.
Important research results include the successful transfer of the production process of nanocrystalline MgH2 as well as NaH-Al composites for hydrogen storage from laboratory mills to using a modified Siebtechnik ESM 236-1bs vibrating mill.
Zoz Simoloyer® CM100-s2 (auto-batch) in our outstation at the ZOZ Technology Centre Olpe, Germany
Current research work also concentrates on the scale-up of light metal hydride and hydride composite production processes to using the ZOZ Simoloyer® CM08 (8 l milling vial) und CM100 (100 l milling vial).
The scientists at the Helmholtz-Zentrum Hereon have been working on the development of hydrogen storage tank systems since 2002.
Within the framework of the EU project “STORHY: Hydrogen Storage Systems for Automotive Applications,” the Helmholtz-Zentrum Hereon in collaboration with the Hamburg University of Technology developed and constructed the first larger hydrogen storage tank in Europe that contains a complex hydride (sodium alanate). We thus managed to demonstrate that the production of storage material using high-energy milling processes can not only be achieved with laboratory mills but also using industrial mills. We could especially establish that such storage tanks based on complex hydrides can be loaded with hydrogen in less than ten minutes.

A Hereon researcher testing a solid state hydrogen storage tank.
With the second generation of these storage tanks, which we developed at the Helmholtz-Zentrum Hereon within the framework of the EU project “NESSHY: Novel Efficient Solid Storage for Hydrogen,” we could demonstrate a gravimetric storage capacity for the tank system of 1.7 % by weight and the potential to reach 2.4% by weight through slight modifications.
To extend beyond this storage tank capacity, materials with even higher storage capacities, such as Reactive Hydride Composites, are necessary.
